Analysis of the Factors Influencing Drugs Pharmacokinetics in Disease State and Their Clinical Relevance
Under the influence of the modern life
styles, diseases show a tendency of becoming complex and chronic. As a
result, long term medical intervention has also become inevitable. In
the treatment of patients, the risk of therapeutic failure or poor control
of the disease is present. This is because in addition to the drug side
effects that will also be experienced by a healthy person who received
similar drug treatment, the pathological state of a disease may evoke
unexpected drug effects can be classified into two category, namely, a
consequence of altered pharmacodynamics, i.e. change in target protein
sensitivity; and a consequence of altered pharmacokinetics, i.e. change
in absorption, distribution, metabolism, and excretion of the drug. Hence,
needless to say that a thorough understanding of the drug pharmacokinetics
under different disease states is extremely important.
In chronic renal failure patients, uremic toxins which are harmful metabolites,
have been found to accumulate to a high degree in occurrence of the uremic
plasma. In order to overcome the complication that inhibits the QOL of
dialysis patients, to prevent the adverse effects of drugs in chronic
renal failure patients, it is necessary to clarify the interaction mechanism
between biogenic molecule and uremic toxins.
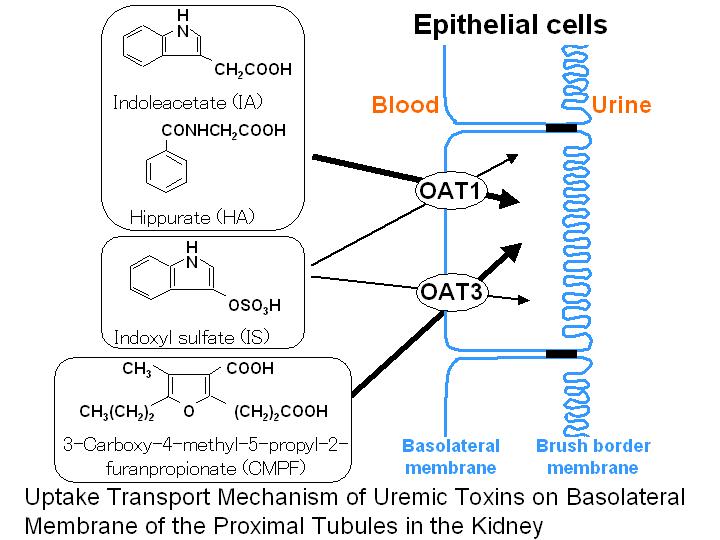
Recently, we have reported that uremic toxins such as indoxyl sulfate,
3-carboxy-4-methyl-5-propyl-2-furanpropionate, indoleacetate and hippurate
play an important role in the inhibition of serum protein binding of drugs
in chronic renal failure. In addition, we have shown in pharmacokinetic
analysis using rats that, these uremic toxins are mainly eliminated from
plasma via active tubular secretion of the kidney. Subsequently, rat organic
anion transporter 1 (rOat1) and rOat3 have been shown to involve in their
uptake on renal basolateral membrane of the proximal tubules using stable
transfectants of rOat1, rOat3 and rat kidney slices. In addition, human
OAT1 (hOAT1) and hOAT3 have also shown to involve in the renal uptake
of uremic toxins. Recent findings suggest that uremic toxins promote the
progression of renal failure by damaging tubular cells . Consequently,
increased concentrations of uremic toxins in the renal tubules would exacerbate
the deterioration of renal function in chronic renal failure, suggesting
that OATs-mediated uptake of these toxins lead to further loss of nephrons.
At present, we are attempting to predict the pharmacological alteration
of drugs and expression mechanism of uremic syndrome from the pharmacokinetic
properties of uremic toxins and pathophysiological condition of the patient.
Research projects of the following topics are currently underway:
Analysis
of the Factors Influencing Drugs Pharmacokinetics in Disease State and
Their Clinical Relevance
1. Molecular Mechanisms of the Tissue Distribution Properties of Uremic
Toxins
2. Functional and Molecular Characterization of Drug Transporters in
Disease State
3. Clinical Therapeutic Strategy Utilizing the Drug-Drug Interaction
Phenomena
4. Stereoselectivity in the Pharmacodynamics
Reference(2000〜)
Shimoishi K, Anraku M, Kitamura K, Tasaki Y, Taguchi K, Hashimoto M,
Fukunaga E, Maruyama T, Otagiri M.
An oral adsorbent, AST-120 protects against the progression of oxidative
stress by reducing the accumulation of indoxyl sulfate in the systemic
circulation in renal failure.Pharm Res. 24:1283-1289 (2007)
Kadowaki D, Anraku M, Tasaki Y, Kitamura K, Wakamatsu S, Tomita K, Gebicki JM, Maruyama T, Otagiri M. Related Articles, Links Effect of olmesartan on oxidative stress in hemodialysis patients. Hypertens Res. 30:395-402 (2007)
Deguchi T, Isozaki K, Yousuke K, Terasaki T, Otagiri M. Involvement of organic anion transporters in the efflux of uremic toxins across the blood-brain barrier. J Neurochem. 96:1051-1059 (2006).
Deguchi T, Takemoto M, Uehara N, Lindup WE, Suenaga A, Otagiri M. Renal clearance of endogenous hippurate correlates with expression levels of renal organic anion transporters in uremic rats. J. Pharmacol. Exp. Ther. 314:932-938 (2005).
Tahara H, Shono M, Kusuhara H, Kinoshita H, Fuse E, Takadate A, Otagiri
M, Sugiyama Y. Molecular cloning and functional analyses of OAT1 and OAT3
from cynomolgus monkey kidney. Pharm Res. 22:647-660 (2005).
Deguchi T, Kouno Y, Terasaki T, Takadate A, Otagiri M. Differential contributions
of rOat1 (Slc22a6) and rOat3 (Slc22a8) to the in vivo renal uptake of
uremic toxins in rats. Pharm Res. 22:619-627 (2005).
Wakida N, Tuyen do G, Adachi M, Miyoshi T, Nonoguchi H, Oka T, Ueda O, Tazawa M, Kurihara S, Yoneta Y, Shimada H, Oda T, Kikuchi Y, Matsuo H, Hosoyamada M, Endou H, Otagiri M, Tomita K, Kitamura K. Mutations in human urate transporter 1 gene in presecretory reabsorption defect type of familial renal hypouricemia. J. Clin. Endocrinol. Metab. 90:2169-2174 (2005).
Takamura N, Maruyama T, Chosa E, Kawai K, Tsutsumi Y, Uryu Y, Yamasaki K, Deguchi T, Otagiri M. Bucolome, a potent binding inhibitor for furosemide, alters the pharmacokinetics and diuretic effect of furosemide: potential for use of bucolome to restore diuretic response in nephrotic syndrome. Drug Metab. Dispos. 33:596-602 (2005).
Ohtsuki S, Kikkawa T, Mori S, Hori S, Takanaga H, Otagiri M and Terasaki
T. Mouse "reduced in osteosclerosis" transporter (Roct) functions
as an organic anion transporter 3 (mOAT3) and is localized at abluminal
membrane of blood-brain barrier. J. Pharmacol. Exp. Ther. 309:1273-1281(2004).
Deguchi T, Kusuhara H, Takadate A, Endou H, Otagiri M and Sugiyama Y.
Characterization of uremic toxin transport by organic anion transporters
in the kidney. Kidney Int. 65:162-174 (2004).
Deguchi T, Nakamura M, Tsutsumi Y, Suenaga A and Otagiri M. Pharmacokinetics
and tissue distribution of uraemic indoxyl sulphate in rats. Biopharm.
Drug Dispos. 24:345-355 (2003).
Imai T, Yoshigae Y, Hosokawa M, Chiba K and Otagiri M. Evidence for the
involvement of a pulmonary first-pass effect via carboxylesterase in the
disposition of a propranolol ester derivative after intravenous administration.
J. Pharmacol. Exp. Ther. 307:1234-1242 (2003).
Imai T, Nomura T and Otagiri M. Probenecid-induced changes in the clearance
of pranoprofen enantiomers. Chirality 15:318-323 (2003).
Imai T, Nomura T, Aso M and Otagiri M. Enantiospecific disposition of
pranoprofen in beagle dogs and rats. Chirality 15:312-317
(2003).
Tsutsumi Y, Deguchi T, Takano M, Takadate A, Lindup WE and Otagiri M.
Renal disposition of a furan dicarboxylic acid and other uremic toxins
in the rat. J. Pharmacol. Exp. Ther. 303:880-887 (2002).
Ohtsuki S, Asaba H, Takanaga H, Deguchi T, Hosoya K, Otagiri M and Terasaki
T. Role of blood-brain barrier organic anion transporter 3 (OAT3) in the
efflux of indoxyl sulfate, a uremic toxin: its involvement in neurotransmitter
metabolite clearance from the brain. J. Neurochem. 83:57-66
(2002).
Imamura H, Komori T, Ismail A, Suenaga A and Otagiri M. Stereoselective
protein binding of alprenolol in the renal diseased state. Chirality
14:599-603 (2002).
Deguchi T, Ohtsuki S, Otagiri M, Takanaga H, Asaba H, Mori S and Terasaki
T. Major role of organic anion transporter 3 in the transport of indoxyl
sulfate in the kidney. Kidney Int. 61:1760-1768 (2002).
Sakai T, Yamasaki K, Sako T, Kragh-Hansen U, Suenaga A and Otagiri M.
Interaction mechanism between indoxyl sulfate, a typical uremic toxin
bound to site II, and ligands bound to site I of human serum albumin.
Pharm. Res. 18:520-524 (2001).
Tsutsumi Y, Maruyama T, Takadate A, Shimada H and Otagiri M. Decreased
bilirubin-binding capacity in uremic serum caused by an accumulation of
furan dicarboxylic acid. Nephron 85:60-64 (2000).